Kinh Nghiệm về When the concentration of a reactant a first order reaction is doubled the rate becomes times but for order reaction the rate remains same? Chi Tiết
Pro đang tìm kiếm từ khóa When the concentration of a reactant a first order reaction is doubled the rate becomes times but for order reaction the rate remains same? được Update vào lúc : 2022-10-21 12:05:22 . Với phương châm chia sẻ Thủ Thuật Hướng dẫn trong nội dung bài viết một cách Chi Tiết 2022. Nếu sau khi đọc nội dung bài viết vẫn ko hiểu thì hoàn toàn có thể lại Comment ở cuối bài để Ad lý giải và hướng dẫn lại nha.All AP Chemistry Resources
Nội dung chính- All AP Chemistry Resources
- All AP Chemistry Resources
- What happens to the reaction rate if the concentration is doubled in a first order reaction?
- What happens to a reaction if concentration is doubled?
- What happens to the reaction rate when the concentrations of both reactants are doubled?
- What will happen to the rate if the concentration of A is doubled and the concentration of B is tripled?
In a third order reaction with two reactants, if you triple the concentration of one of the reactants, the rate increases by a factor of 3. What happens to the rate of the reaction if the concentration of the second reactant is halved?
Possible Answers:
It decreases by a factor of 4.
It doubles.
It is halved.
It increases by a factor of 4.
Correct answer:
It decreases by a factor of 4.
Explanation:
Based on the question, you can tell that the rate is first order with respect to the first reactant. So if the overall reaction is third-order, that means that the exponents must add to 3. We know that the exponent of the first reactant is 1, so that must mean that the exponent of the second reactant is 2. Thus, the concentration will be squared when you account for how it contributes to the rate, so the rate increases by a facotor of 4.
Given the following. What is the rate law for the reaction A + 2B → C
[A](M) [B] (M) Rate (M/s)
0.1 0.02 0.005
0.2 0.02 0.02
0.1 0.01 0.0025
Possible Answers:
Rate = k [B]
Rate = k [A] [B]
Rate = k [A]2[B]
Rate = k [A]
Rate = k [A] [B]2
Correct answer:
Rate = k [A]2[B]
Explanation:
The rate is only based on the experimental values in the table. When B doubles and A stays the same the rate doubles, making it first order with respect to compound B. When compound A doubles and B stays the same, the rate quadruples, making it second order in regards to A.
The rate of the reaction 2A + B → C was measured different concentrations of reactants. The results are as follows:
[A] [B] Rate
0.5 2 0.1
0.5 4 0.4
1 2 0.2
Possible Answers:
r = k[A]2[B]
r = k[B]
r = k[A][B]2
r = k[A][B]
r = k[A]
Correct answer:
r = k[A][B]2
Explanation:
When the concentration of A stays the same but the concentration of B doubles, the rate quadruples, showing a second order rate law based on B. When the concentration of A doubles and B stays the same, the rate also doubles, showing a first order rate law based on A.
A rate law __________.
Possible Answers:
can always be derived from looking the reaction in question
can never be completely independent of the concentrations of any of the reactants
always requires experimental data to determine
is measured in unit of concentration over time
Correct answer:
is measured in unit of concentration over time
Explanation:
Rate laws can rarely be determined from just the reaction; they usually require experimental data. However, both of the answer choices contain the word ALWAYS, which is too extreme. Rate laws can be independent of the reactants, these rate laws are known as zero-order.
Consider the following balanced equation.
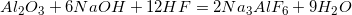
The rate law for this reaction is .
What is the rate constant for this reaction if the reaction proceeds an initial rate of when all of the reactants have initial concentrations of
Correct answer:
Explanation:
Since the rate law is provided for the reaction, we can plug in the values that are relevant to the rate of the reaction. This will allow us to determine the rate constant.
Note that HF is not included; the reaction must be zero order with respect to HF.
Consider the following reaction and experimental data.
Trial
[A] (M)
[B] (M)
Initial reaction rate
(M/s)
1
0.02
0.03
0.005
2
0.02
0.09
0.005
3
0.04
0.09
0.020
What is the rate law for this reaction?
Correct answer:
Explanation:
When determining the rate law for a reaction, you need to determine the order for each reactant in the reaction. This can only be accomplished by performing an experiment where different trials show how the initial reaction rate changes based on the initial concentrations of the reactants. By keeping one initial reactant concentration constant and changing the concentration of the other reactant, we can see how the initial rate changes for each reactant.
For reactant A, we notice that the rate quadruples when its concentration is doubled by comparing trials 2 and 3. The concentration of A increases from 0.02 to 0.04, causing the rate to change from 0.005 to 0.020. This means that the order for reactant A is 2.
For reactant B, we see that the rate does not change when the initial concentration of B is tripled by comparing trials 1 and 2. As a result, the reaction is 0 order wih respect to B.
Now that we have the orders for each reactant, we can write the rate law accordingly.
Since anything to the 0 power is 1, B is omitted from the rate law and can be considered to be equal to 1.
You want to find the order of each reactant by manipulating the reactant concentrations in multiple trials. The table below shows the effect of altered reactant concentrations on initial reaction rate.
Using the above trials, write the rate law for the reaction.
Correct answer:
Explanation:
Keep in mind that the chemical equation and its coefficients have NOTHING to do with the rate law. The order of each reactant must be determined by experiment.
To find the order of each reactant, compare the initial reaction rates of two trials in which only one of the three reactants' concentrations is altered. For example, trials 1 and 4 keep A and B equal, but C is doubled. When C is doubled, we see that the initial reaction rate is quadrupled. As a result, we determine that the order of reactant C is 2. When the reactant is altered, but the initial reaction rate is kept constant, as seen in trials 1 and 3 with respect to A, the order of that reactant is 0. Finally, when the reactant is multiplied by the same factor that the initial reaction rate is multiplied, as seen in trials 1 and 2 with respect to B, the order of the reactant is 1.
Putting the data together: A is zeroth order, B is first order, and C is second order. Our rate law can thus be written .
Given the following equation (2A+B --> 3C). Which of the following correctly displays the rate of the reaction?
I. -Δ[A]/2Δt
II. Δ[B]/Δt
III. Δ[C]/3Δt
Possible Answers:
I and III only
I, II, and III
II only
I only
II and III only
Correct answer:
I and III only
Explanation:
The rate based on concentration is related to the coefficients in front of the compounds. Based on the reactants the rate should be negative (because change in concentration for the forward reaction will be negative) and based on the products should be positive. This means that II is incorrect. The rate for each compound in the reaction should be divided by it's coefficient to make it all related to 1M, showing that I and III are correct.
For the following reaction H2 + 2 ICl → 2 HCl + I2 describe the rate of consumption
of H2 in regards to the
consumption of ICl and the production of I2
Possible Answers:
Same as ICl, same as I2
Half of ICl, half of I2
Half of ICl, same as I2
Same as ICl, half of I2
Correct answer:
Half of ICl, same as I2
Explanation:
By the stoichiometry, since 1 mole of of H2 and 2 moles of ICl produces 2 moles of HCl
and 1 mole of I2 , we know that H2 is consume half as
fast as ICl and produced the same
rate as I2 .
Which of the following is a classic example of a first-order reaction?
Possible Answers:
None of the other answers
Radioactive decay
A change in temperature
A collision between 2 reactant molecules
Correct answer:
Radioactive decay
Explanation:
First order reactions have rates that are directly proportional to only 1 reactant. In radioactive decay, the rate of decrease of a radioactive material is proportional only to the amount of the material.
All AP Chemistry Resources
What happens to the reaction rate if the concentration is doubled in a first order reaction?
When you double the concentration the rate doubles. The rate is proportional to the square of the concentration of a reactant.What happens to a reaction if concentration is doubled?
If the concentration is doubled, the rate will quadruple (second order reaction). : rate = k. If the concentration is doubled, the rate will stay the same (zeroth order reaction).What happens to the reaction rate when the concentrations of both reactants are doubled?
For the reaction A+B→C+D, doubling the concentration of both the reactants increases the reaction rate by 8 times and doubling the concentration of only B simply doubles the reaction rate.What will happen to the rate if the concentration of A is doubled and the concentration of B is tripled?
First, we have to write the given rate law. Then, we will triple the concentration of A and double the concentration of B. Hence, the reaction rate would be increased by a factor of 12. Tải thêm tài liệu liên quan đến nội dung bài viết When the concentration of a reactant a first order reaction is doubled the rate becomes times but for order reaction the rate remains same? Reply
Reply
 4
4
 0
0
 Chia sẻ
Chia sẻ
Chia Sẻ Link Cập nhật When the concentration of a reactant a first order reaction is doubled the rate becomes times but for order reaction the rate remains same? miễn phí
Bạn vừa tìm hiểu thêm tài liệu Với Một số hướng dẫn một cách rõ ràng hơn về Review When the concentration of a reactant a first order reaction is doubled the rate becomes times but for order reaction the rate remains same? tiên tiến và phát triển nhất và Chia SẻLink Tải When the concentration of a reactant a first order reaction is doubled the rate becomes times but for order reaction the rate remains same? miễn phí.